Nuclear Magnetic Resonance Spectroscopy
Background
Nuclear Magnetic Resonance (NMR) is not easy to describe in a few paragraphs, let alone understand. Therefore, this description of NMR takes a simplistic point of view. There is much more to NMR than is contained here.
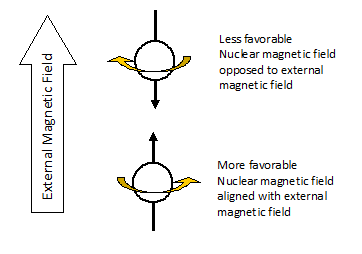
The nuclei of certain atoms (hydrogen, for example) behave as if they are spinning like tiny, positively-charged tops. A spinning nucleus (because it is charged) generates its own magnetic field. If we place a sample containing these nuclei in an external magnetic field (between the poles of a powerful magnet), the magnetic field of the spinning nuclei can either be aligned with, or opposed to, the external magnetic field. It is more favorable for the nuclear magnetic field to be aligned with the external magnetic field, but if the right amount of energy is supplied, it can be forced into the opposed state.
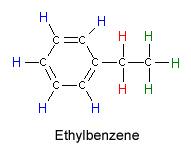
Ethylbenzene showing hydrogen atoms in three different environments
The nucleus can change its orientation from the aligned to the opposed state if it absorbs the right energy of radiofrequency radiation. The size of the energy difference between the two orientations depends on the strength of the magnetic field that the nucleus feels. Because molecular structures can shield or expose the atom from the external magnetic field, the energy of radiation absorbed provides information about the location of that nucleus within the molecule. In addition, adjacent nuclei interact to give us information about which nuclei are near one another. As an example, you might consider the hydrogen atoms in an ethylbenzene molecule.
Older NMR instruments worked by bombarding a sample with 60 MHz radiation (just below the end of your FM dial), and increasing the magnetic field to differentiate between hydrogen atoms in a molecule. In the example here, the magnetic field strength increases from left to right. When a hydrogen nucleus feels the right magnetic field to absorb the 60 MHz radiation, we see a peak. Hydrogen atoms in different parts of the molecule require different field strengths to absorb that specific energy. Using this approach it is possible to identify the hydrogen atoms on the ring, and those attached to each carbon atom.
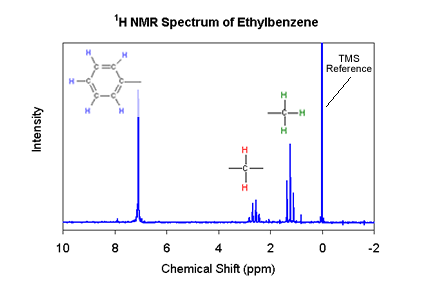
NMR spectrum of Ethylbenzene
NMR Instrumentation
Anasazi Eft 60 Fourier Transform NMR Spectrometer
The Anazazi Eft 60 is considered a low-field strength NMR. We believe it is a great tool to teach students the fundamentals of NMR spectroscopy. Students get their start acquiring and interpreting NMR results in their sophomore year in organic chemistry courses. While we primarily use this instrument for proton NMR spectra, it is capable of detecting hydrogen, carbon-13, fluorine-19, silicon-29 and phosphorous-31, and can execute sophisticated two-dimensional correlation techniques such as COSY and HETCOR.
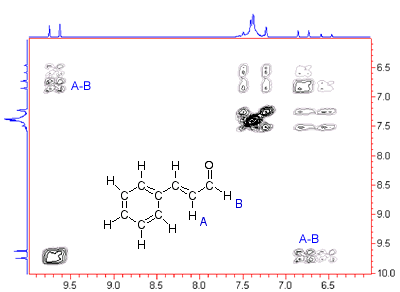
The figure at left is a COSY(H-H Correlation Spectroscopy), a two-dimensional NMR spectrum that gives information about what hydrogens within the molecule are close to one another. The peaks labeled A-B, for example, indicate that hydrogens A and B in the structure are on adjacent carbons.
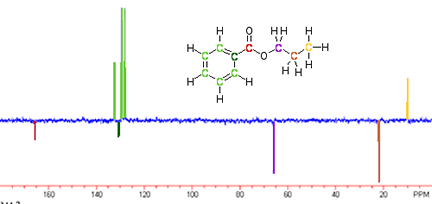
The adjacent spectrum is an example of a carbon-13 NMR. The reason it has both positive and negative peaks is that it is the result of an Attached Proton Test. This spectrum not only gives information about the electronic environment surrounding the carbons in our sample molecule, but it also indicates how many hydrogens are attached to each carbon. The peaks pointing down correspond to carbons with 0 or 2 hydrogens attached. The peaks pointing up correspond to carbons with 1 or 3 hydrogens attached.